The 100 000 Genomes Project is a ground-breaking study which will bring the power of new genetic technologies to NHS patients. Eleven Genomic Medicine Centres (GMCs) from around England will be (or are already) recruiting patients with either a cancer or a rare genetic disorder. These patients will go on to have their entire genomes sequenced. The first patient was recruited to the study earlier this month.
A company called Genomics England, owned by the Department of Health, has been set up to run the project. To run alongside it, a Masters programme in Genomic Medicine is being set up in 6-8 sites in England. One of the participating Genomic Medicine Centres (SouthWest Peninsula) is our very own Royal Devon and Exeter NHS Foundation Trust.
The goal of the project is to transform the NHS, bringing genetics to mainstream medicine, allowing for the development of new therapies for cancer and rare disorders. The term for this is "precision medicine". Genetic information has the capability to predict which individuals will respond best to which cancer therapeutic agent, which drug side effects they may be at risk of developing, and the sub-type of rare genetic disorder which they have.
Patients who are eligible to participate in this project are either those with a recent diagnosis of one of a defined set of cancers (for example, breast cancer, lung cancer; there are several others); or, secondly, a rare disorder (examples: a congenital malformation, a rare syndrome with learning disability, or a rare neurological disorder). There are literally thousands of rare disorders, individually very rare, but collectively common - Rare Disease UK found that 1 in 17 individuals in the UK is affected by a rare disorder. Participating GMCs are able to 'nominate' specific rare disorders which Genomics England will then consider and, if appropriate, approve.
There are valid reasons for scepticism about the project. Here are a few:
At yet another recent meeting, this time of clinicians and laboratory scientists, we had a talk from a bioinformatician. These people have the job of converting the raw genome sequence into something that is accessible to and usable by the clinician. I asked him, I thought playfully, whether or not there was in his view a prospect that people could in the future download their genome sequence onto their mobile phones and analyse them on an 'app' ("MyGenome"?). He couldn't see an issue with the genome download, and no doubt the clever people at Google, Apple and elsewhere are in the process of developing the app right now. As technical challenges go, I wouldn't have thought it would be harder than some of the other stuff they do- driverless cars, for example.
In the 100 000 Genomes Project, participants can request to receive their own raw genome data in addition to any results. As at 2015, the process for this has not yet been defined by Genomics England, but there is no reason to think that it shouldn't be possible, even though there will probably have to be a payment.
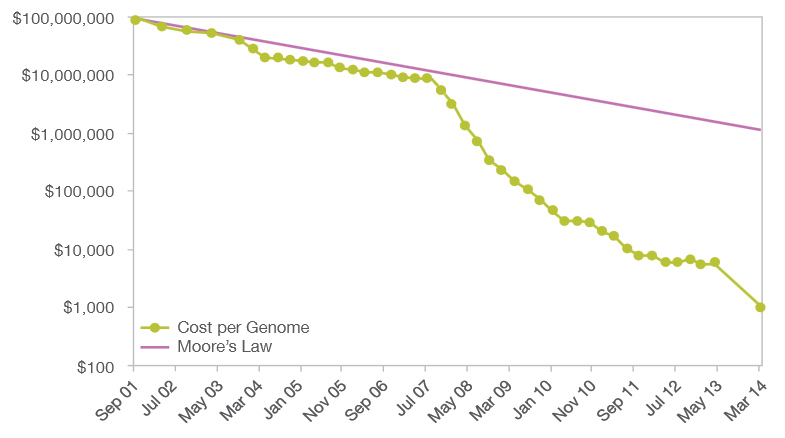
Outside the 100 000 Genomes Project, the more interesting question is to ask at which point people from the population at large will start to want to have access to their own genome data. As always, a key element of this question (not the only one, of course) is cost. Here is a graph of the cost of sequencing a genome over time, to the present, taken from this source:
Once the cost of a whole genome sequence falls to a value which is accessible to the private citizen, and the technology to look at it is developed into a user-friendly interface, then the private citizen is likely to look carefully at the value of knowing this information. Yes, of course there are millions of variants of uncertain or low significance. But it is the handful of variants of very well known, and extremely adverse, clinical significance, that people could possibly want to know about.
If people could find out very easily, and for an essentially trivial cost, that they were at, say, 80% risk of developing breast or bowel cancer in their lifetime; or that, as a couple, they were at high risk of having a child with a pre- or post-natally lethal disorder, would they ignore this opportunity? I wonder.
Views expressed in this article are my own and not those of Peninsula Regional Genetics Service, Royal Devon and Exeter Hospital, SouthWest Peninsula Genomic Medicine Centre, Genomics England or any other public body.
A company called Genomics England, owned by the Department of Health, has been set up to run the project. To run alongside it, a Masters programme in Genomic Medicine is being set up in 6-8 sites in England. One of the participating Genomic Medicine Centres (SouthWest Peninsula) is our very own Royal Devon and Exeter NHS Foundation Trust.
The goal of the project is to transform the NHS, bringing genetics to mainstream medicine, allowing for the development of new therapies for cancer and rare disorders. The term for this is "precision medicine". Genetic information has the capability to predict which individuals will respond best to which cancer therapeutic agent, which drug side effects they may be at risk of developing, and the sub-type of rare genetic disorder which they have.
Patients who are eligible to participate in this project are either those with a recent diagnosis of one of a defined set of cancers (for example, breast cancer, lung cancer; there are several others); or, secondly, a rare disorder (examples: a congenital malformation, a rare syndrome with learning disability, or a rare neurological disorder). There are literally thousands of rare disorders, individually very rare, but collectively common - Rare Disease UK found that 1 in 17 individuals in the UK is affected by a rare disorder. Participating GMCs are able to 'nominate' specific rare disorders which Genomics England will then consider and, if appropriate, approve.
There are valid reasons for scepticism about the project. Here are a few:
- The issue of consent is a complex one, made more so by the fact that patients will be asked whether or not they wish to receive 'secondary' (also called 'unlooked for' findings). These findings relate to their future health but are unrelated to the medical reason for which they were enrolled in the project, for example, a fault in a gene conferring susceptibility to breast and ovarian cancer identified in a child with a rare neurological disorder.
- The IT infrastructure requirement for the project is significant and will need considerable investment of time, energy and money- previous efforts to reform NHS IT do not necessarily give grounds for optimism
- There are issues regarding data security. Patients who enrol in the project will be asked to consent to release of clinical information and genomic data to third parties, which will include commercial as well as academic groups, for the purposes of research and development. This idea also has some tricky past history, still far from resolved.
At yet another recent meeting, this time of clinicians and laboratory scientists, we had a talk from a bioinformatician. These people have the job of converting the raw genome sequence into something that is accessible to and usable by the clinician. I asked him, I thought playfully, whether or not there was in his view a prospect that people could in the future download their genome sequence onto their mobile phones and analyse them on an 'app' ("MyGenome"?). He couldn't see an issue with the genome download, and no doubt the clever people at Google, Apple and elsewhere are in the process of developing the app right now. As technical challenges go, I wouldn't have thought it would be harder than some of the other stuff they do- driverless cars, for example.
In the 100 000 Genomes Project, participants can request to receive their own raw genome data in addition to any results. As at 2015, the process for this has not yet been defined by Genomics England, but there is no reason to think that it shouldn't be possible, even though there will probably have to be a payment.
Outside the 100 000 Genomes Project, the more interesting question is to ask at which point people from the population at large will start to want to have access to their own genome data. As always, a key element of this question (not the only one, of course) is cost. Here is a graph of the cost of sequencing a genome over time, to the present, taken from this source:

Once the cost of a whole genome sequence falls to a value which is accessible to the private citizen, and the technology to look at it is developed into a user-friendly interface, then the private citizen is likely to look carefully at the value of knowing this information. Yes, of course there are millions of variants of uncertain or low significance. But it is the handful of variants of very well known, and extremely adverse, clinical significance, that people could possibly want to know about.
If people could find out very easily, and for an essentially trivial cost, that they were at, say, 80% risk of developing breast or bowel cancer in their lifetime; or that, as a couple, they were at high risk of having a child with a pre- or post-natally lethal disorder, would they ignore this opportunity? I wonder.
Views expressed in this article are my own and not those of Peninsula Regional Genetics Service, Royal Devon and Exeter Hospital, SouthWest Peninsula Genomic Medicine Centre, Genomics England or any other public body.
No comments:
Post a Comment